

学习资源
科普文章
克服癌症中CD3+ T细胞重定向相关的挑战(二):TCE与激动剂联用策略[1]
双特异性抗体的开发将CD3+ T细胞的细胞毒活性重定向到肿瘤是治疗血液系统恶性肿瘤和实体癌的一种有前途的免疫治疗策略。自2014年底FDA批准抗CD3 ×抗CD19双特异性抗体blinatumomab (Blincyto®)治疗复发/难治性b细胞急性淋巴细胞白血病以来,已经启动了约100项研究CD3+双特异性T细胞定向器治疗癌症的安全性和有效性的临床试验。然而,尽管早期取得了成功,但癌症背景下CD3+ T细胞重定向存在许多挑战,包括“反作用”CD3+ T细胞亚群的招募和系统性细胞因子释放。小编在这次推荐的综述总结了CD3双抗和免疫共抑制/刺激靶点调节剂的临床联用策略[1]。上篇文章中已经涉及TCE与PD-1抗体联用,感兴趣的读者可点击链接,克服癌症中CD3+ T细胞重定向相关的挑战[1]
这里我们将重点介绍TCE与共刺激抗体的联用。
CD3+双特异性T细胞重定向结合免疫检查点共刺激剂
虽然拮抗T细胞上的共抑制受体已被证明是增强CD3+ T细胞定向器(TCE)抗肿瘤活性的一种有前景的方法,但也有证据表明,能够激活T细胞上的共刺激受体的激动性抗体,包括肿瘤坏死因子受体(TNFR)超家族成员(例如,4-1BB, OX40, GITR, CD27)或免疫球蛋白超家族成员(例如,CD28, ICOS),可用于增强对CD3+ T细胞参与疗法的免疫反应。值得注意的是,在黑素瘤小鼠模型中,一种激动性抗4- 1BB抗体被证明可以恢复耗尽的CD8+肿瘤浸润淋巴细胞的功能[2]。同样,在B16F10-OVA黑色素瘤小鼠模型中,用激动性抗4-1BB抗体刺激4-1BB信号通路导致细胞毒性T淋巴细胞的持续时间延长及其功能增强[3]。抗-4- 1BB共刺激也被证明可以在体外和前列腺癌同基因小鼠模型中增强抗CD3 ×抗PSMA双特异性抗体的抗肿瘤活性(图1和图2)[4]。
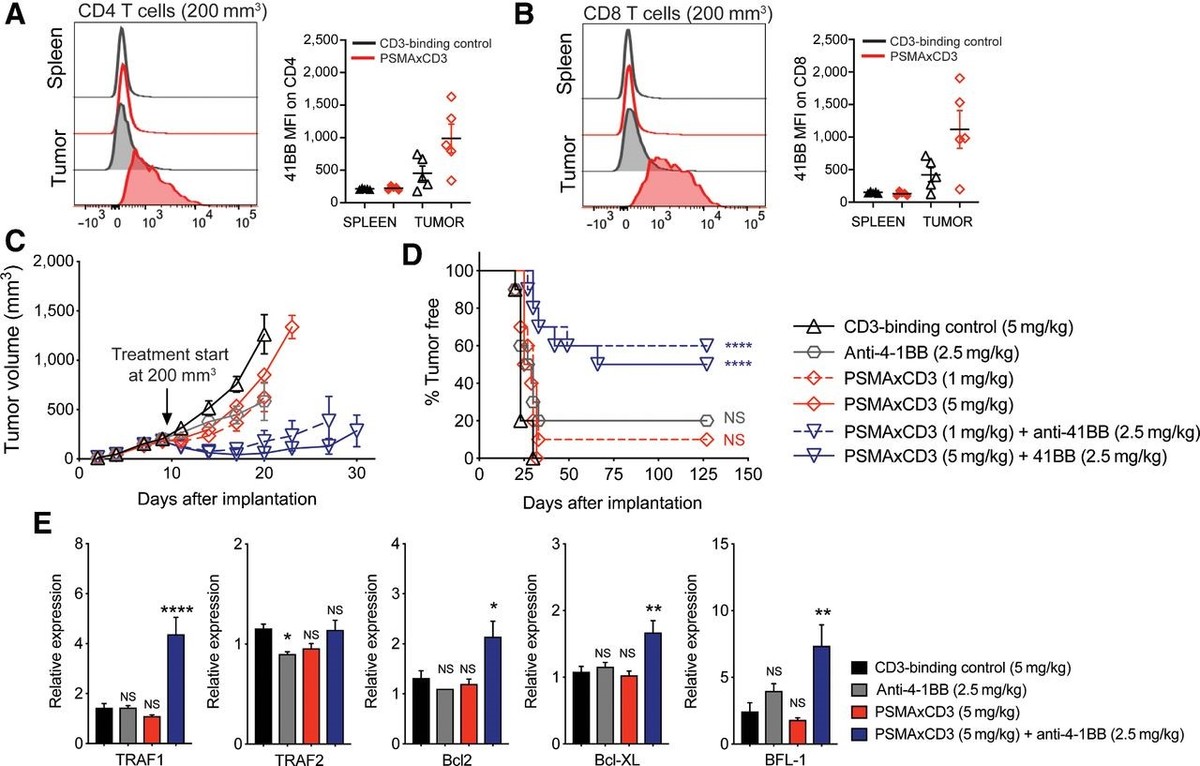
Figure 1. PSMAxCD3 treatment combined with 4-1BB costimulation is efficacious against larger tumors. A, Representative flow plots and MFI of 4-1BB expression on splenic and tumor CD4+ T cells in HuT mice (n = 5) with large 200 mm3 tumors treated with 5 mg/kg of CD3-binding control or PSMAxCD3 48 hours after dose. B, Representative flow plots and MFI of 4-1BB expression on splenic and tumor CD8+ T cells in HuT mice (n = 5) with large 200 mm3 tumors treated with 5 mg/kg of CD3-binding control or PSMAxCD3 48 hours after dose. Established 200 mm3 TRAMP-C2-hPMSA tumors were treated once on day 9 (arrow) with 5 mg/kg CD3-binding control, 2.5 mg/kg anti-4-1BB, 1 mg/kg PSMAxCD3, 5 mg/kg PSMAxCD3, 1 mg/kg PSMA with 2.5 mg/kg anti-4-1BB, or 5 mg/kg PSMAxCD3 with 2.5 mg/kg anti-4-1BB. C, Mean tumor volume is shown as SEM (n = 10, 3 replicates). Average tumor growth curve is plotted until first mouse of each group was euthanized. D, Survival curve represents tumor-free mice. Significance is measured by Gehan–Breslow–Wilcoxon test compared with CD3-binding control (****, P < 0.0001). E, Relative expression of 4-1BB pathway genes in tumors 72 hours after dose was measured by TaqMan. Data are shown as SEM (n = 6; ****, P < 0.0001; **, P < 0.01; *, P < 0.05). Statistical significance measured by one-way ANOVA compared with CD3-binding control. MFI, mean fluorescence intensity; NS, not significant.
图1. PSMAxCD3 治疗结合 4-1BB 共刺激对较大的肿瘤有效。A,具有 200 mm3 大肿瘤的 HuT 小鼠(n = 5)脾脏和肿瘤 CD4+ T 细胞上 4-1BB 表达的代表性流图和 MFI 5 mg/kg 的 CD3 结合对照或 PSMAxCD3 给药后 48 小时。B,具有 200 mm3 大肿瘤的 HuT 小鼠(n = 5)脾脏和肿瘤 CD8+ T 细胞上 4-1BB 表达的代表性流图和 MFI 5 mg/kg 的 CD3 结合对照或 PSMAxCD3 给药后 48 小时。已建立的 200 mm3 TRAMP-C2-hPMSA 肿瘤在第 9 天(箭头)用 5 mg/kg CD3 结合对照、2.5 mg/kg 抗 4-1BB、1 mg/kg 治疗一次 PSMAxCD3、5 mg/kg PSMAxCD3、1 mg/kg PSMA 和 2.5 mg/kg 抗 4-1BB,或 5 mg/kg PSMAxCD3 和 2.5 mg/kg 抗 4-1BB。C,平均肿瘤体积显示为 SEM(n = 10,重复 3 次)。绘制平均肿瘤生长曲线,直到每组的第一只小鼠被安乐死。D,生存曲线代表无肿瘤小鼠。与 CD3 结合对照相比,通过 Gehan–Breslow–Wilcoxon 检验测量显着性(****,P < 0.0001)。E,通过 TaqMan 测量给药后 72 小时肿瘤中 4-1BB 通路基因的相对表达。数据显示为 SEM(n = 6;****,P < 0.0001;**,P < 0.01;*,P < 0.05)。与 CD3 结合对照相比,通过单向方差分析测量的统计显着性。MFI,平均荧光强度;NS,不显着。
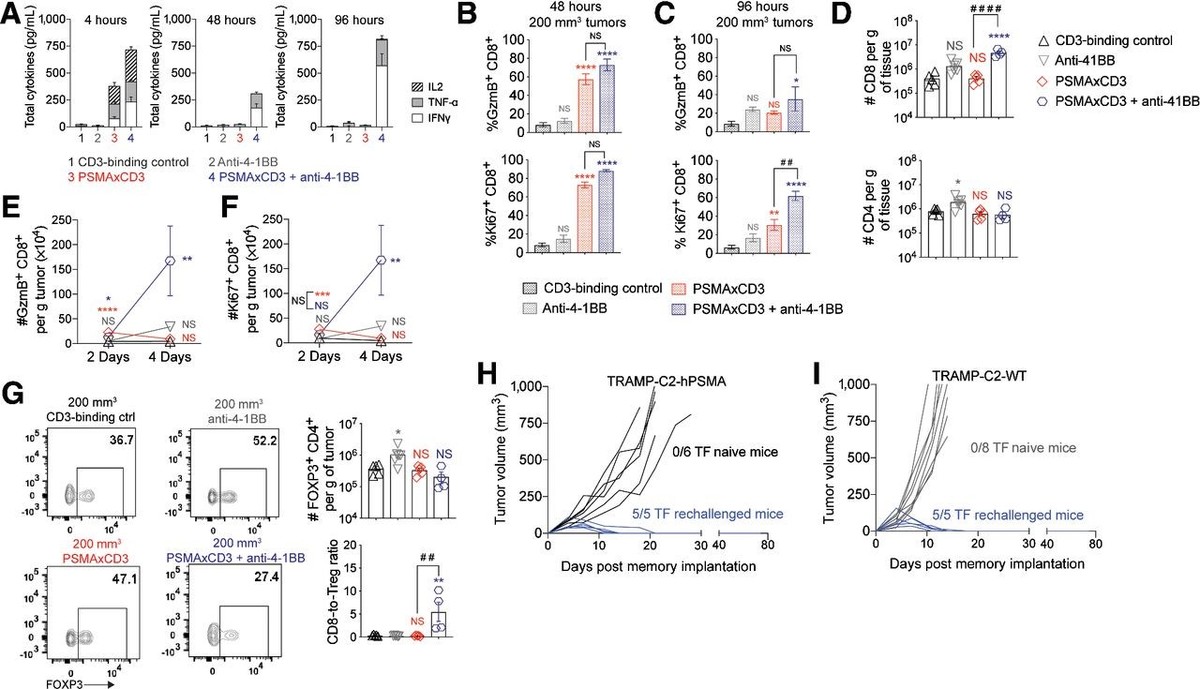
Figure 2. PSMAxCD3 with 4-1BB costimulation in mice increases expansion and activation of CD8+ T cells. A, HuT mice are treated with 5 mg/kg of CD3-binding control, anti-4-1BB, or PSMAxCD3 with and without 2.5 mg/kg anti-4-1BB (n = 5). Serum cytokine concentrations were measured by MSD at 4, 48, and 96 hours after treatment administration. B, Percentages of granzyme B+ or Ki67+ CD8+ T cells 48 hours after treatment examined by flow cytometry. C, Percentages of granzyme B+ or Ki67+ CD8+ T cells 96 hours after treatment examined by flow cytometry. D, Total counts of CD8+ and CD4+ T cells were examined at 96 hours after treatment by flow cytometry. E, Total counts of tumor-infiltrating granzyme B+ CD8+ T cells after treatment administration examined by flow cytometry. F, Total counts of tumor-infiltrating Ki67+ CD8+ T cells after treatment administration examined by flow cytometry. G, Representative flow plots, total counts of FOXP3+ CD4+ T cells, and CD8+-to-Treg ratios 96 hours after treatment administration. All statistical significance is measured by one-way ANOVA compared with CD3-binding control (****, P < 0.0001, ***; P < 0.001; **, P < 0.01; *, P < 0.05) or PSMAxCD3 compared with PSMAxCD3 with anti-4-1BB (####, P < 0.0001; ##, P < 0.01). Mice that cleared the 200 mm3 tumors were rechallenged. H, Individual tumor growth curves from mice reimplanted with TRAMP-C2-hPSMA cells 35 days after initial tumor clearance. I, Individual tumor growth curves of TRAMP-C2 cells that were implanted 50 days after initial tumor clearance. NS, not significant; TF, tumor-free.
图 2. PSMAxCD3 在小鼠中与 4-1BB 共刺激增加了 CD8+ T 细胞的扩增和激活。A,HuT 小鼠用 5 mg/kg 的 CD3 结合对照、抗 4-1BB 或 PSMAxCD3 处理,有和没有 2.5 mg/kg 抗 4-1BB(n = 5)。在治疗给药后 4、48 和 96 小时,通过 MSD 测量血清细胞因子浓度。B,处理后 48 小时通过流式细胞仪检查的颗粒酶 B+ 或 Ki67+ CD8+ T 细胞的百分比。C,处理后 96 小时通过流式细胞术检查的颗粒酶 B+ 或 Ki67+ CD8+ T 细胞的百分比。D,在处理后 96 小时通过流式细胞术检测 CD8+ 和 CD4+ T 细胞的总计数。E,通过流式细胞术检查治疗给药后肿瘤浸润性颗粒酶 B+ CD8+ T 细胞的总计数。F,通过流式细胞仪检测治疗给药后肿瘤浸润性 Ki67+ CD8+ T 细胞总数。G,治疗给药后 96 小时的代表性流程图、FOXP3+ CD4+ T 细胞总数和 CD8+-to-Treg 比率 . 与 CD3 结合对照(****,P < 0.0001,***;P < 0.001;**,P < 0.01;*,P < 0.05)或 PSMAxCD3 比较,通过单向方差分析测量所有统计显着性 PSMAxCD3 和抗 4-1BB(####,P < 0.0001;##,P < 0.01)。清除 200 mm3 肿瘤的小鼠再次接受攻击。H,在初始肿瘤清除后 35 天,来自重新植入 TRAMP-C2-hPSMA 细胞的小鼠的个体肿瘤生长曲线。I,初始肿瘤清除后 50 天植入的 TRAMP-C2 细胞的个体肿瘤生长曲线。NS,不显着;TF,无肿瘤。
此外,成纤维细胞活化蛋白(FAP)-4-1BBL与CEA靶向T细胞双特异性抗体联合治疗,CD19-4-1BBL与CD20靶向T细胞双特异性抗体联合治疗均导致小鼠模型中肿瘤显著消退,肿瘤内聚集活化的CD8+细胞毒性T细胞。机制上,在T细胞受体信号传递存在的情况下,肿瘤抗原靶向的4-1BB激动剂诱导4-1BB响应是通过与肿瘤抗原介导的交联实现的,而不依赖FcγR介导的交联 (图3-6) [5]。
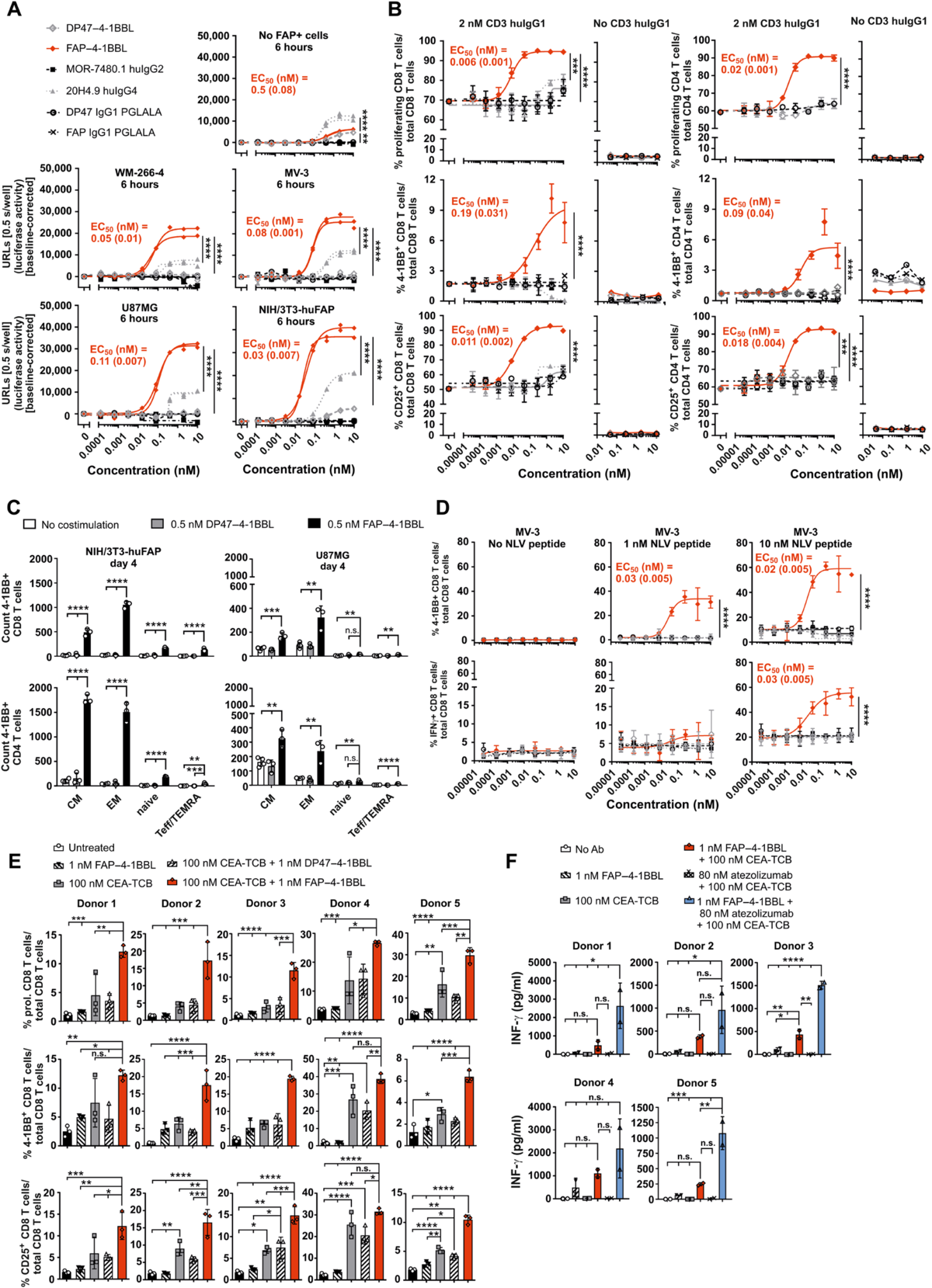
Fig. 3. FAP–4-1BBL induces in vitro T cell activation in the presence of signal 1. (A) HeLa-hu4-1BB-NFκB-luc reporter cells were cocultured with or without huFAP-expressing cells, 4-1BB agonists, or control proteins. Luciferase activity was measured as units of released light (URLs) (n = technical duplicates). Significance was calculated, comparing the area under the curve (AUC) by unpaired one-way analysis of variance (ANOVA) with Tukey’s multiple comparison test. EC50 values for FAP–4-1BBL are indicated as mean (SD). (B) Carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled human peripheral blood mononuclear cells (huPBMCs) were cocultured with irradiated NIH/3T3-huFAP fibroblasts in the presence or absence of agonistic 2 nM CD3 huIgG1 and titrated 4-1BB agonists. Proliferation (CFSE dilution), 4-1BB, and CD25 expression of CD4+ or CD8+ T cells after 5 days [mean (SD), n = technical triplicates] are shown. Significance was calculated as in (A). (C) CFSE-labeled huPBMCs were cocultured with irradiated FAP-expressing cells (NIH/3T3-huFAP or U87MG), 2 nM CD3 huIgG1 alone, or in combination with DP47–4-1BBL or FAP–4-1BBL. CD4+ and CD8+ T cells were characterized for their expression of CD45RO, CD45RA, and CD62L by flow cytometry. Total counts of 4-1BB–expressing T cells divided in CM, EM, naive, and Teff/TEMRA subpopulations are shown [mean (SD), n = technical triplicates]. Unpaired one-way ANOVA with Tukey’s multiple comparison test was used. (D) CFSE-labeled HLA-A*02:01/NLV peptide–specific EM CD8+ T cells were cocultured with NLV peptide pulsed (1 or 10 nM) or unpulsed HLA-A*02:01+ FAP+ MV-3 melanoma cells and 4-1BB agonists or controls. After 24 hours, brefeldin A and monesin were added for 4 hours and cells were analyzed for 4-1BB and IFN-γ expression by flow cytometry [mean (SD), n = technical triplicates]. Significance was calculated as in (A). (E and F) CFSE-labeled huPBMCs of five different donors were cocultured with irradiated NIH/3T3-huFAP and carcinoembryonic antigen (CEA)+ PD-L1+ gastric tumor cells (MKN45-huPD-L1), FAP–4-1BBL, carcinoembryonic antigen-targeted T cell bispecific antibody (CEA-TCB) or atezolizumab alone or in combination [atezolizumab shown only in (F)]. After 4 days, proliferation (CFSE dilution), CD25, and 4-1BB expression of CD8+ T cells [mean (SD), n = technical triplicates] were analyzed by flow cytometry. IFN-γ in supernatant was measured by multiplex (n = technical duplicates). Significance was analyzed as in (C). Each shown experiment represents results of at least three independent and similar experiments. For huPBMC-based assays, at least three different donors were tested, but one representative donor is shown. n.s., not significant.
图 3. FAP–4-1BBL 在信号 1 存在的情况下诱导体外 T 细胞活化。(A) HeLa-hu4-1BB-NFκB-luc 报告细胞与或不与表达 huFAP 的细胞、4-1BB 激动剂或对照蛋白共培养。荧光素酶活性以释放光 (URL) 为单位进行测量(n = 技术重复)。计算显着性,通过未配对的单向方差分析 (ANOVA) 与 Tukey 的多重比较检验比较曲线下面积 (AUC)。FAP–4-1BBL 的 EC50 值表示为平均值 (SD)。(B) 在存在或不存在激动性 2 nM CD3 huIgG1 和滴定 4-1BB 激动剂的情况下,将羧基荧光素二乙酸琥珀酰亚胺酯 (CFSE) 标记的人外周血单核细胞 (huPBMC) 与受照射的 NIH/3T3-huFAP 成纤维细胞共培养。显示了 5 天后 CD4+ 或 CD8+ T 细胞的增殖(CFSE 稀释)、4-1BB 和 CD25 表达 [平均值 (SD),n = 技术一式三份]。如(A)中计算显着性。(C) CFSE 标记的 huPBMC 与受照射的 FAP 表达细胞(NIH/3T3-huFAP 或 U87MG)、2 nM CD3 huIgG1 单独或与 DP47–4-1BBL 或 FAP–4-1BBL 联合培养。通过流式细胞术表征 CD4+ 和 CD8+ T 细胞的 CD45RO、CD45RA 和 CD62L 表达。显示了分为 CM、EM、幼稚和 Teff/TEMRA 亚群的表达 4-1BB 的 T 细胞的总数 [平均值 (SD),n = 技术一式三份]。使用未配对的单向方差分析和 Tukey 的多重比较检验。(D) CFSE 标记的 HLA-A*02:01/NLV 肽特异性 EM CD8+ T 细胞与 NLV 肽脉冲(1 或 10 nM)或未脉冲 HLA-A*02:01+ FAP+ MV-3 黑色素瘤细胞共培养 和 4-1BB 激动剂或对照。24 小时后,加入布雷菲德菌素 A 和莫尼菌素 4 小时,并通过流式细胞术分析细胞的 4-1BB 和 IFN-γ 表达 [平均值 (SD),n = 技术一式三份]。如(A)中计算显着性。(E 和 F)五个不同供体的 CFSE 标记的 huPBMC 与受照射的 NIH/3T3-huFAP 和癌胚抗原 (CEA)+ PD-L1+ 胃肿瘤细胞 (MKN45-huPD-L1)、FAP–4-1BBL、癌胚抗原共培养 抗原靶向 T 细胞双特异性抗体 (CEA-TCB) 或单独或联合使用阿特珠单抗 [阿特珠单抗仅在 (F) 中显示]。4 天后,通过流式细胞术分析 CD8+ T 细胞的增殖(CFSE 稀释)、CD25 和 4-1BB 表达 [平均值 (SD),n = 技术一式三份]。上清液中的 IFN-γ 通过多重测量(n = 技术重复)。如 (C) 中分析显着性。每个显示的实验代表至少三个独立且相似的实验的结果。对于基于 huPBMC 的测定,至少测试了三个不同的供体,但显示了一个具有代表性的供体。n.s.,不重要。
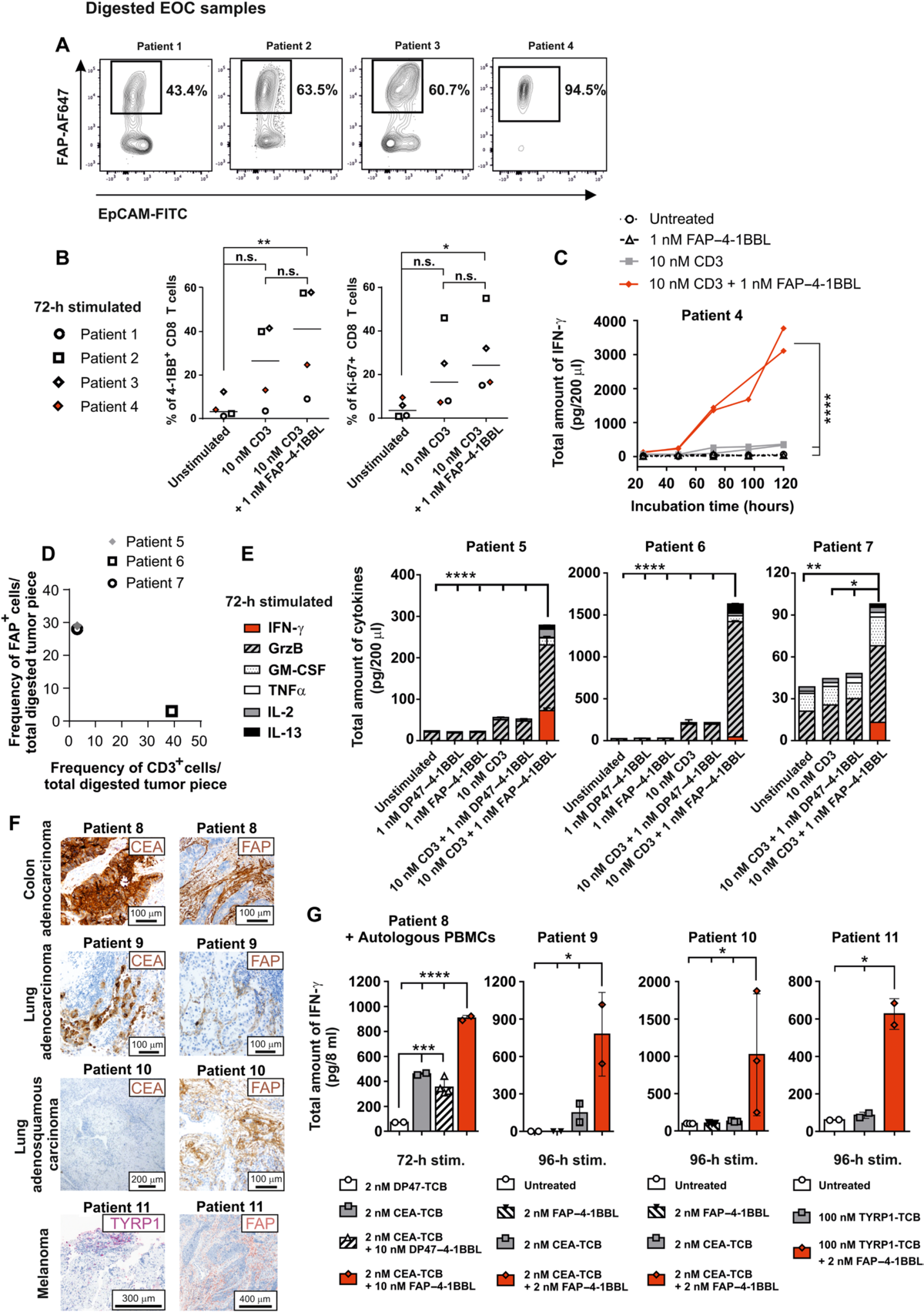
Fig. 4. FAP–4-1BBL in combination with CD3 activation induces cytokine release from FAP-expressing human cancer samples. (A) Single-cell suspensions of EOC tumor lesions were tested for FAP expression. (B) Single-cell suspensions of EOC patients’ tumor tissue were cultured unstimulated, with agonistic CD3 huIgG1 alone, or in combination with FAP–4-1BBL. Shown are the frequencies of 4-1BB+ and Ki67+ CD8+ T cells determined by flow cytometry. Significance was calculated using paired one-way ANOVA with Tukey’s multiple comparison test. (C) Tumor suspension of patient 4 was incubated in medium, with CD3 huIgG1 or FAP–4-1BBL alone or in combination. Supernatant was analyzed for cytokines by multiplex. Technical duplicates are shown as individual curves. Significance was calculated, comparing the AUC using unpaired one-way ANOVA with Tukey’s multiple comparison test. (D) Melanoma digests from three patients were tested for frequency of FAP and CD3-expressing cells by flow cytometry. (E) FAP+ tumor digests were cultured unstimulated or with CD3 huIgG1, FAP–4-1BBL, or DP47–4-1BBL alone or in combination. Secreted cytokines were analyzed using cytometric bead array (CBA). Depending on tumor size sample, n = 2 (patient 5 and 6) or n = 1 (patient 7) technical replicate per condition was measured. Significance was calculated using unpaired twoway ANOVA with Tukey’s multiple comparison test. (F) Colon adenocarcinoma, lung adenocarcinoma, lung adenosquamous carcinoma, or melanoma tumor lesions were analyzed for FAP, CEA, or tyrosinase-related protein (TYRP1) expression. (G) Fresh tumor tissue from lesions shown in (F) were placed in bioreactors (each symbol represents one bioreactor), supplied with 8 ml of medium/reactor, and for patient 8, with autologous huPBMCs. Bioreactors were incubated with medium alone (untreated) or stimulatory molecules as indicated. Supernatant was analyzed for IFN-γusing CBA. Significance was analyzed using unpaired one-way ANOVA multiple comparison, uncorrected Fisher’s LSD test. Because of the limited available patient material, all performed assays are shown.
图 4. FAP–4-1BBL 结合 CD3 激活诱导表达 FAP 的人类癌症样本释放细胞因子。(A) 测试 EOC 肿瘤病变的单细胞悬液的 FAP 表达。(B) EOC 患者肿瘤组织的单细胞悬浮液未经刺激培养,单独使用激动性 CD3 huIgG1,或与 FAP–4-1BBL 联合使用。显示的是通过流式细胞术测定的 4-1BB+ 和 Ki67+ CD8+ T 细胞的频率。使用配对单向方差分析和 Tukey 的多重比较检验计算显着性。(C) 患者 4 的肿瘤悬浮液在培养基中孵育,单独或组合使用 CD3 huIgG1 或 FAP–4-1BBL。通过多重分析上清液的细胞因子。技术重复显示为单独的曲线。计算显着性,将使用未配对的单向方差分析的 AUC 与 Tukey 的多重比较检验进行比较。(D) 通过流式细胞术检测来自三名患者的黑色素瘤消化物的 FAP 和 CD3 表达细胞的频率。(E) FAP+ 肿瘤消化物未经刺激或与 CD3 huIgG1、FAP–4-1BBL 或 DP47–4-1BBL 单独或组合培养。使用流式微珠阵列 (CBA) 分析分泌的细胞因子。根据肿瘤大小样本,测量了每种情况下的 n = 2(患者 5 和 6)或 n = 1(患者 7)技术复制。使用未配对的双向方差分析和 Tukey 的多重比较检验计算显着性。(F) 分析结肠腺癌、肺腺癌、肺腺鳞癌或黑色素瘤肿瘤病变的 FAP、CEA 或酪氨酸酶相关蛋白 (TYRP1) 表达。(G) 来自 (F) 中所示病变的新鲜肿瘤组织被置于生物反应器中(每个符号代表一个生物反应器),提供 8 ml 培养基/反应器,对于患者 8,提供自体 huPBMC。如所示,生物反应器与单独的培养基(未处理的)或刺激分子一起孵育。使用 CBA 分析上清液中的 IFN-γ。使用未配对的单向方差分析多重比较、未校正的 Fisher LSD 检验分析显着性。由于可用的患者材料有限,因此显示了所有执行的化验。
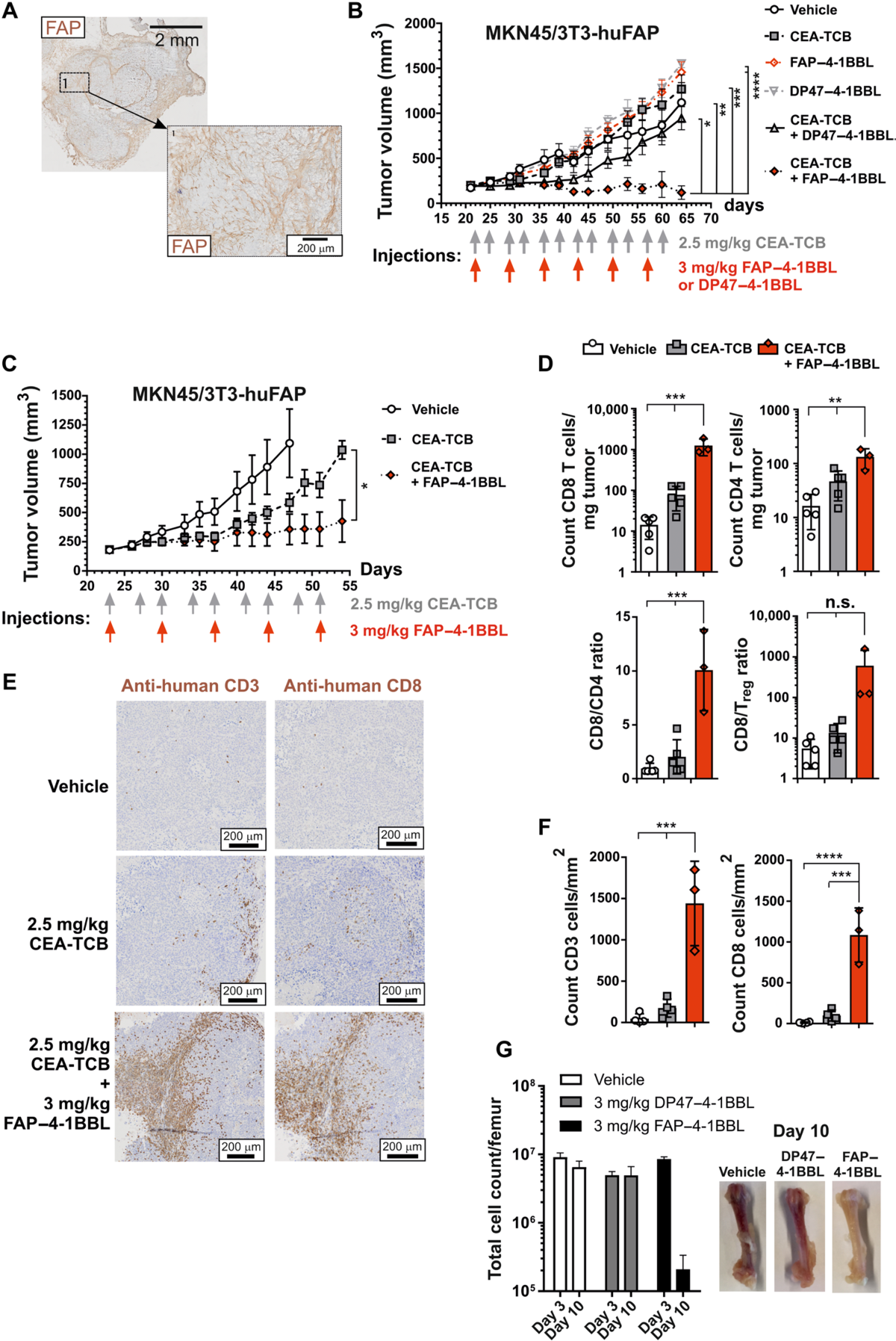
Fig. 5. Combination of FAP–4-1BBL and CEATCB in vivo decreases tumor growth and increases intratumoral CD8+ T cell accumulation. (A) A representative example of FAP expression in the tumor at the start of therapy (200 mm3). (B and C) Shown are two of three independent experiments. CEA+ human gastric cancer cells MKN45 and NIH/3T3-huFAP fibroblasts were coinjected subcutaneously into human stem cell–engrafted NSG (HSC-NSG) mice. At an average tumor size of 200 mm3, mice were treated with vehicle, CEA-TCB, FAP–4-1BBL, or DP47–4-1BBL alone or in combination as indicated (n = 10 per group). Shown is mean (SEM). Statistical significance of tumor volumes at the end point was calculated using unpaired one-way ANOVA with
Tukey’s multiple comparison test (B) or unpaired, two-tailed Student’s t test (C). (D) Digested tumor tissues (day 52) were analyzed by flow cytometry gating on living human CD4+ or CD8+ T cells. Regulatory T (Treg) cells were defined as FoxP3+ CD25+ CD4+ T cells. Each symbol represents one individual mouse; bars indicate mean (SD). Significance was calculated using unpaired oneway ANOVA with Tukey’s multiple comparison test. (E) Representative immunohistochemistry staining for CD3+ and CD8+ cells is shown. Tissue sections were scanned, and whole scans were analyzed by Definiens. Tumors were taken from the experiment shown in (C) at termination. (F) CD3+ and CD8+ T cells count per square millimeter of immunohistochemistry analyzed by Definiens for the whole section. Each symbol represents one individual mouse; shown is mean (SD). Significance was calculated as in (D). (G) Ten HSC-NSG mice per group were treated weekly with vehicle, FAP–4-1BBL, or DP47–4-1BBL intravenously. Three days after first (day 3) and second injection (day 10), bone marrow from femur (n = 5 per group) was analyzed for cell counts by flow cytometry. Tumor experiments were repeated in three independent experiments; bone marrow evaluation experiment was assessed once.
图 5. FAP–4-1BBL 和 CEATCB 在体内的组合降低了肿瘤生长并增加了肿瘤内 CD8+ T 细胞的积累。(A) 治疗开始时肿瘤中 FAP 表达的代表性示例 (200 mm3)。(B 和 C) 显示的是三个独立实验中的两个。CEA+ 人胃癌细胞 MKN45 和 NIH/3T3-huFAP 成纤维细胞被皮下注射到人干细胞移植的 NSG (HSC-NSG) 小鼠体内。在平均肿瘤大小为 200 mm3 时,小鼠单独或联合使用载体、CEA-TCB、FAP–4-1BBL 或 DP47–4-1BBL 进行治疗(每组 n = 10)。显示的是平均值 (SEM)。使用未配对的单向方差分析计算终点肿瘤体积的统计显着性
Tukey 的多重比较检验 (B) 或未配对的双尾学生 t 检验 (C)。(D) 通过对活人 CD4+ 或 CD8+ T 细胞设门的流式细胞术分析消化的肿瘤组织(第 52 天)。调节性 T (Treg) 细胞定义为 FoxP3+ CD25+ CD4+ T 细胞。每个符号代表一只小鼠;条形表示平均值 (SD)。使用未配对的单向方差分析和 Tukey 的多重比较检验计算显着性。(E) 显示了 CD3+ 和 CD8+ 细胞的代表性免疫组织化学染色。组织切片被扫描,整个扫描由 Definiens 分析。在终止时从 (C) 中所示的实验中取出肿瘤。(F) Definiens 对整个切片分析的每平方毫米免疫组织化学 CD3+ 和 CD8+ T 细胞计数。每个符号代表一只小鼠;显示的是平均值 (SD)。如(D)中计算显着性。(G) 每组 10 只 HSC-NSG 小鼠每周接受载体、FAP–4-1BBL 或 DP47–4-1BBL 静脉内治疗。第一次(第 3 天)和第二次注射(第 10 天)后三天,通过流式细胞术分析股骨骨髓(每组 n = 5)的细胞计数。在三个独立的实验中重复肿瘤实验;骨髓评价实验评价一次。
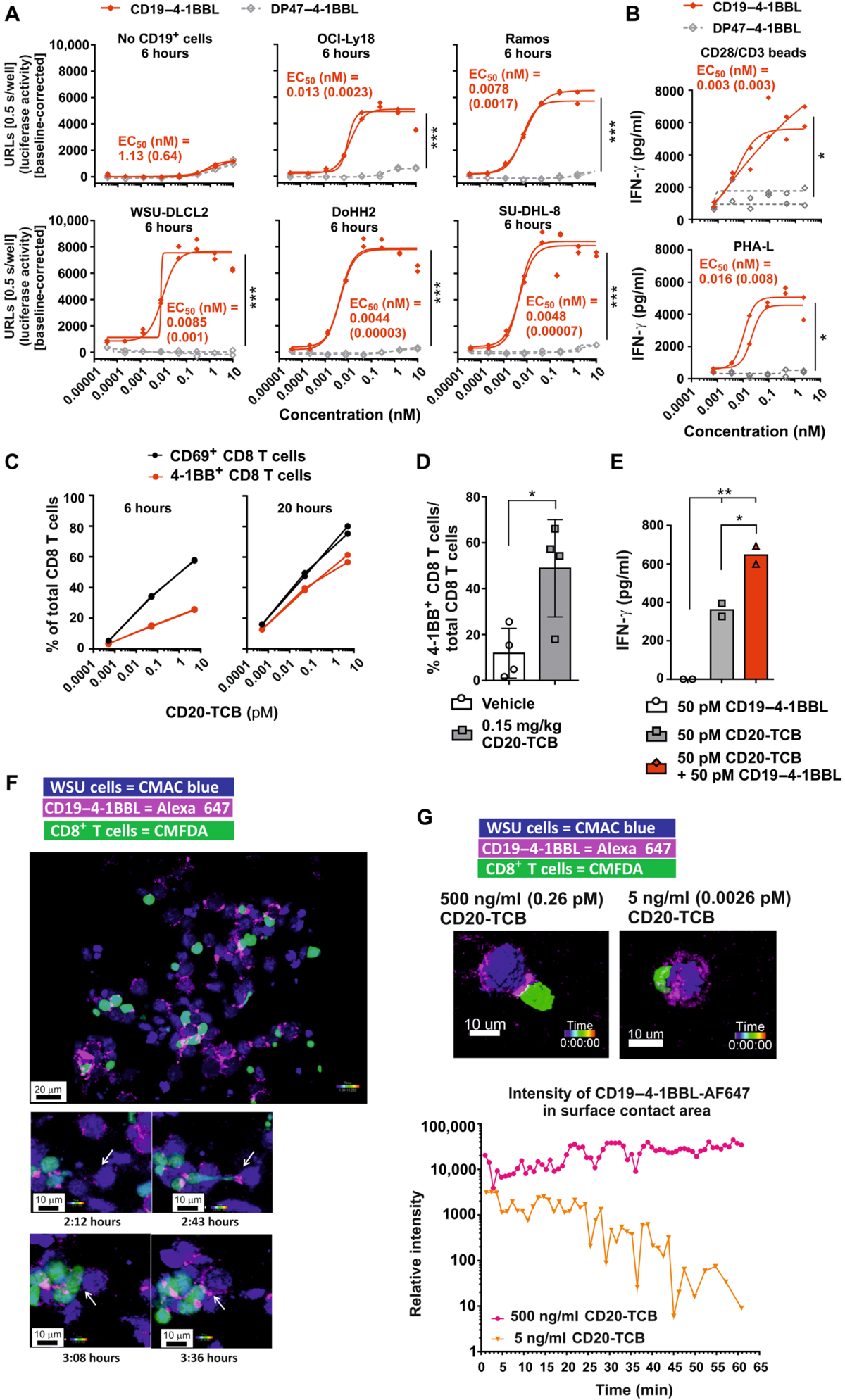
Fig. 6. CD19–4-1BBL induces in vitro T cell activation and localizes with CD20-TCB in the immunological synapse. (A) HeLa-hu4-1BBNFκB-luc reporter cells were cocultured in the absence or presence of huCD19-expressing cell lines and CD19–4-1BBL or DP47–4-1BBL. Shown is the luciferase activity after 6 hours as URLs (n = technical duplicates). Significance was calculated comparing AUC by unpaired two-tailed Student’s t test. EC50 values are indicated as mean (SD). (B) huPBMCs (n = technical duplicates) were incubated with 1.2 × 106 CD3/CD28 beads/ml (top) or PHA-L (1μg/ml) (bottom) and CD19–4- 1BBL or DP47–4-1BBL, whereby CD19–4-1BBL is cross-linked via B cells. IFN-γsecretion was analyzed by ELISA after 2 days. Significance was calculated as in (A), and EC50 values are indicated as mean (SD). (C) Human CD3+ T cells isolated from buffy coat (n = technical duplicates) were cocultured with WSU-DLCL2 cells and CD20-TCB. Expression of 4-1BB and CD69 on CD8+ T cells was determined by flow cytometry. (D) WSU-DLCL2–bearing HSC-NSG mice (n = 4) were treated weekly intravenously with vehicle or CD20-TCB. Tumors were isolated 2 days after second injection, and tumor-infiltrating CD8+ T cells were analyzed by flow cytometry for 4-1BB expression. Each symbol indicates one individual mouse. Shown is mean (SD); significance was calculated by unpaired two-tailed Student’s t test. (E) CD3+ T cells were cocultured with WSU-DLCL2 cells, CD19–4-1BBL, and CD20-TCB as indicated. After 18 hours, supernatants were analyzed by ELISA for IFN- secretion. Shown is the mean (SD) of n = technical duplicates, indicated with symbols. Significance was calculated using an unpaired one-way ANOVA with Tukey’s multiple comparison test. (F) Localization of CD19–4-1BBL (magenta), WSU-DLCL2 cells (blue), and activated 4-1BB–expressing CD8+ T cells (green) by confocal fluorescence microscopy. (G) The localization of CD19–4-1BBL, WSU-DLCL2 cells, and CD20-TCB–activated 4-1BB–expressing CD8+ T cells was monitored for up to 90 frames. In the graph, the intensity of AF647-labeled CD19–4-1BBL in the surface contact area between 4-1BB+ CD8+ T cells and CD19+ WSU-DLCL2 cells is shown. Experiments shown in (A) to (D) were repeated at least three times in independent experiments. If primary cells were used, then at least three different donors were tested. Experiments shown in (E) to (G) were performed only once.
图 6. CD19–4-1BBL 诱导体外 T 细胞活化并定位于免疫突触中的 CD20-TCB。(A) HeLa-hu4-1BBNFκB-luc 报告细胞在表达 huCD19 的细胞系和 CD19-4-1BBL 或 DP47-4-1BBL 存在或不存在的情况下共培养。显示的是 6 小时后的荧光素酶活性作为 URL(n = 技术重复)。通过未配对的双尾学生 t 检验比较 AUC 来计算显着性。EC50 值表示为平均值 (SD)。(B) huPBMC(n = 技术重复)与 1.2 × 106 CD3/CD28 珠/ml(顶部)或 PHA-L (1μg/ml)(底部)和 CD19-4-1BBL 或 DP47-4-1BBL 一起孵育, 由此 CD19–4-1BBL 通过 B 细胞交联。2 天后通过 ELISA 分析 IFN-γ 分泌。如 (A) 中计算显着性,EC50 值表示为平均值 (SD)。(C) 从血沉棕黄层分离的人 CD3+ T 细胞(n = 技术重复)与 WSU-DLCL2 细胞和 CD20-TCB 共培养。通过流式细胞术测定 4-1BB 和 CD69 在 CD8+ T 细胞上的表达。(D) 携带 WSU-DLCL2 的 HSC-NSG 小鼠 (n = 4) 每周接受载体或 CD20-TCB 静脉内治疗。第二次注射后 2 天分离肿瘤,并通过流式细胞术分析肿瘤浸润 CD8+ T 细胞的 4-1BB 表达。每个符号表示一只单独的小鼠。显示的是平均值 (SD);通过未配对的双尾学生 t 检验计算显着性。(E) 如图所示,CD3+ T 细胞与 WSU-DLCL2 细胞、CD19–4-1BBL 和 CD20-TCB 共培养。18 小时后,通过 ELISA 分析上清液的 IFN- 分泌。显示的是 n = 技术重复的平均值 (SD),用符号表示。使用未配对的单向方差分析和 Tukey 的多重比较检验计算显着性。(F) 通过共聚焦荧光显微镜定位 CD19–4-1BBL(洋红色)、WSU-DLCL2 细胞(蓝色)和激活的表达 4-1BB 的 CD8+ T 细胞(绿色)。(G) CD19–4-1BBL、WSU-DLCL2 细胞和 CD20-TCB 激活的表达 4-1BB 的 CD8+ T 细胞的定位被监测多达 90 帧。在图中,显示了 4-1BB+ CD8+ T 细胞和 CD19+ WSU-DLCL2 细胞之间的表面接触区域中 AF647 标记的 CD19-4-1BBL 的强度。(A) 至 (D) 中所示的实验在独立实验中至少重复三次。如果使用原代细胞,则至少测试三个不同的供体。(E) 至 (G) 中所示的实验仅进行了一次。
与4-1BB一样,其他共刺激分子如CD28已被证明可以改善对CD3+ T细胞重定向抗体的抗肿瘤免疫反应。用抗CD3 ×抗肿瘤抗原和抗CD28 ×抗肿瘤抗原交叉作用的双特异性抗体治疗癌细胞,可增强体外肿瘤细胞依赖性T细胞激活[6]。此外,抗CD28 ×抗PSMA和抗CD28 ×抗黏蛋白16 (MUC16)双特异性抗体分别在人免疫前列腺癌和卵巢癌小鼠模型中增强CD3+双特异性T细胞重定向器的体内抗肿瘤活性[7]。重要的是,联合疗法在食蟹猴体内没有引起毒性。这些结果表明,患者T细胞或肿瘤细胞上的共刺激(和共抑制)分子的表达谱可以为CD3+ T细胞重定向器的潜在联合治疗提供信息。
总的来说,CD3+双特异性T细胞定向器用于癌症免疫治疗的发展已经取得了实质性进展。多种血液学和实体肿瘤靶点已被用于CD3+ T细胞重定向,该治疗类别中约100种抗体的安全性和有效性目前正处于临床研究阶段。然而,与CD3+ T细胞重定向相关的一些挑战可能会限制抗肿瘤疗效。由于这些挑战在其他癌症免疫治疗方法(如CAR-T细胞治疗)中很常见,从这些研究中获得的经验教训可以潜在地应用于CD3+ T细胞重定向。癌症免疫治疗领域的未来方向可能包括确定与CD3+双特异性T细胞重定向剂的最佳联合疗法,以增强抗肿瘤免疫,同时最大限度地减少毒性。
本文仅作信息分享,不代表礼进生物公司立场和观点,也不作治疗方案推荐和介绍。如有需求,请咨询和联系正规医疗机构。
参考文献
1.Singh, A., S. Dees, and I.S. Grewal, Overcoming the challenges associated with CD3+ T-cell redirection in cancer. British Journal of Cancer, 2021. 124(6): p. 1037-1048.
2.Williams, J.B., et al., The EGR2 targets LAG-3 and 4-1BB describe and regulate dysfunctional antigen-specific CD8+ T cells in the tumor microenvironment. J Exp Med, 2017. 214(2): p. 381-400.
3.Weigelin, B., et al., Focusing and sustaining the antitumor CTL effector killer response by agonist anti-CD137 mAb. Proc Natl Acad Sci U S A, 2015. 112(24): p. 7551-6.
4.Chiu, D., et al., A PSMA-Targeting CD3 Bispecific Antibody Induces Antitumor Responses that Are Enhanced by 4-1BB Costimulation. Cancer Immunol Res, 2020. 8(5): p. 596-608.
5.Claus, C., et al., Tumor-targeted 4-1BB agonists for combination with T cell bispecific antibodies as off-the-shelf therapy. Science Translational Medicine, 2019. 11(496): p. eaav5989.
6.Willems, A., et al., CD3 x CD28 cross-interacting bispecific antibodies improve tumor cell dependent T-cell activation. Cancer Immunol Immunother, 2005. 54(11): p. 1059-71.
7.Skokos, D., et al., A class of costimulatory CD28-bispecific antibodies that enhance the antitumor activity of CD3-bispecific antibodies. Science Translational Medicine, 2020. 12(525): p. eaaw7888.







 沪公网安备 31011502015333号
沪公网安备 31011502015333号